A recent study published in the Journal of Clinical Psychopharmacology found that an FDA cleared, computer-based, objective measurement of ADHD symptoms was more sensitive to medication effects than patient self-rating, at both one-month and six-month follow-up visits.
“Investigating the Clinical Utility of the Combined Use of Objective and Subjective Measures of ADHD During Treatment Optimization” found that QbTest serves as a better early indicator of treatment effects for ADHD, confirming that healthcare professionals can confidently use objective data alongside patient feedback regarding treatment effectiveness.
Clinical ADHD treatment procedures typically involve self-rated evaluations of symptoms as a means for measuring medication effects in adults with ADHD. Yet, most patients find it difficult to assess improvements after pharmacological treatment. The results of the new study indicate that the QbTest offers healthcare providers and others diagnosing and treating ADHD a way to use objective data to make better-informed treatment decisions.
QbTest has proven to show highly sensitive measures of treatment effect
At the first follow up, QbTest’s activity and inattention measures showed significant effect of medication, compared to ASRS, that indicated only moderate effect. Additionally, after six months, the QbTest captured 86% of patient’s treatment effects compared with only 37% of patient’s improvement captured by the ASRS.
Correlations between the QbTest and ASRS were low to moderate at baseline and follow-up visits, in line with earlier studies. The associations between the QbTest and subjective measures increased over time, and importantly, improvements on both measures correlated with improvement in quality-of-life scores.
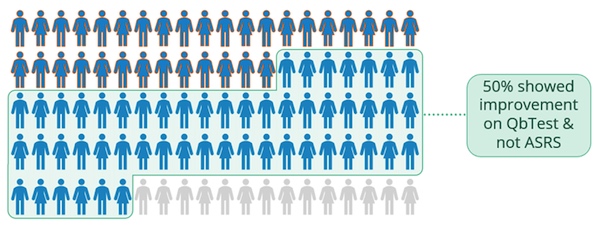
The ability to capture improvement in twice as many patients supports the inclusion of objective testing when making treatment decisions for ADHD patients, and these results suggest that the QbTest provides an early indicator of treatment effect.
“QbTest has proven to show highly sensitive measures of treatment effect, which could lead to better treatment decisions, use of other therapies, and the ability to avoid unnecessarily high doses of stimulant medication,” says Dr. Perry Roy. “The study proves how important it is to have objective data when managing ADHD symptoms.”
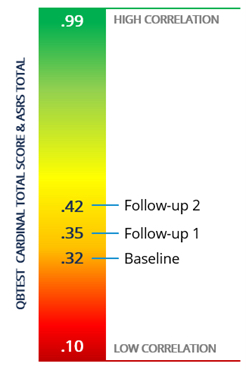
The magnitude of the relationship between rating scales and QbTest increased over time, with more alignment at six months than baseline or initial follow-up, as represented by the image to the left.
This may be explained by the under reporting of symptoms, and increased awareness of their own symptoms over the course of their treatment.
This study’s results are in line with a previous study showing that 54% of adult ADHD patients’ self-ratings were less sensitive to treatment effects than QbTest. Results of both of these studies indicate the need for a comprehensive view of ADHD’s impact on patients. This view would best be served to include both an objective and subjective perspective on all three core symptoms of ADHD to enhance the clinician’s ability to make treatment decision.
So why is this important to know?
As a clinician, if your patient reported minimal effect after adhering to their treatment for six months, would you increase the dose? Would you change the type of treatment? If the decision to modify medication was made, this research indicates that the changes in treatment could be unnecessary approximately 50% of the time.
By adding objective ADHD testing like QbTest into your current evaluation methods, you will be provided with robust data on your patients’ symptomatology to help guide decisions around titration and efficacy to achieve optimal patient outcomes. Having those test results to share with your patients helps support your own judgement and provide the patient with additional reasoning for your decision, therefore improving psychoeducation.
Want to find out more about the research?